What Is the Electron Geometry of So3
Hybridization of SO3 Sulphur Trioxide A detailed description of the hybridization of SO 3 is provided on this page along with its molecular geometry. Sulfur trioxide has a trigonal planar electron geometry according to David Roth of Tutoring Homework Help.

Draw The Lewis Structure For So3 Determine The Number Of Electron Groups The Electron Geometry And The Molecular Shape Is It Polar Or Nonpolar Study Com
The molecular geometry of S O 3 2 is a trigonal pyramidal structure with bond angles of 1 0 7.
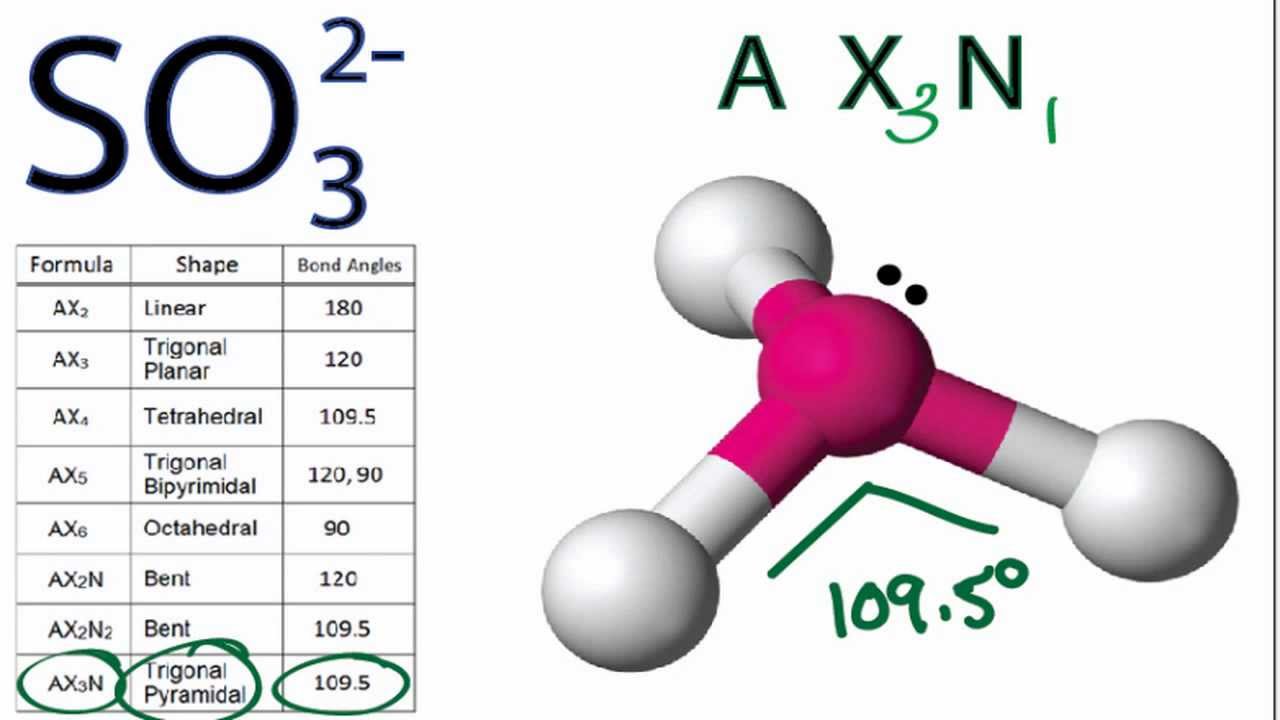
. Whats the electron geometry of SO3. Want to see the step-by-step answer. Molecular and electron geometry of so3-2.
The electron domain and molecular geometry of SO3 S O 3 are. In terms of electron-counting formalism the sulfur atom has an oxidation state of 6 and a formal charge of 0. What is the electron domain geometry of SO3.
The central sulfur atom of SO3 has an oxidation state of 6 and a formal charge. This problem has been solved. The center sulfur atom of SO3 has zero lone pair of electron resulting in trigonal planar SO3 electron geometry.
As a result the SO3 molecule is nonpolar. The Lewis Dot Structure for SO3 2-. Is ClF3 a polar nonpolar or ionic compound.
Name of the Molecule. Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes. Trigonal bipyramidal trigonal planar.
SO 3 has a sp 2 type of hybridization. Two oxygens form single bonds with sulfur while one forms a double bond. What is the electron geometry of SO3.
What is the electron domain geometry of PCl5. I believe that electronic geometry or electronic arrangement refers to the shape of the molecule regarding the positions of electron density while molecular geometry or shape only takes actual atoms into account not the electron density of a lone pair for example. We review their content and use your feedback to keep the quality high.
Trigonal planar trigonal planar. The ion SO3 2- is pyramidal. As an example a sulfate ion SO3 2- has three oxygen atoms bonded to.
We will understand how the molecule obtains such hybridized state below. The electron domain and molecular geometry of SO3 are ________. Molecular structure and bonding Gaseous SO3 is a trigonal planar molecule of D3h symmetry as predicted by VSEPR theory.
SO3 belongs to the D3h point group. A trigonal bipyramidal T-shaped B octahedral seesaw C trigonal planar trigonal planar D trigonal bipyramidal trigonal planar E trigonal planar bent. What is the electron domain geometry of CH4.
Molecular Geometry of Sulfur Trioxide SO3 The above image clears that the bond angle among oxygen-sulfur-oxygen O-S-O atoms have to be more than 90. 2- 0--S--O O the molecule SO3 is triangular with resonance. Experts are tested by Chegg as specialists in their subject area.
Who are the experts. However the molecular geometry of SO3 looks trigonal planar shaped and zero lone pair of electron on the sulfur of the SO3 geometry. Chemistry questions and answers.
The Lewis structure consists of an SO. What is the molecular geometry of BrF5. Check out a sample QA here.
Its trigonal planar and it exhibits resonanceSO3s simplified Lewis structure looks something like thisOSOO. Which statement is true about the energy levels of bonding and antibonding orbitals. Its the SO3 molecules symmetrical geometry.
Is NH3 a polar nonpolar or ionic compound. Molecular geometry of SO3. The accepted Lewis structure predicts both single and double bonds between S and O atoms involving available valence electrons.
SO3 2- is a polar molecule. As a result the SO3 molecule is nonpolar. Its the SO3 molecules symmetrical geometry.
How to find SO3 hybridization and molecular geometry Calculating lone pairs of electrons on sulfur. Trigonal planar tetrahedral seesaw bent check_circle Expert Answer. It is a polar molecule because it has dipole moments due to its trigonal pyramidal geometry.
What is the electron domain geometry of SF6. The sulfite anion SO3 2- is present in wines and is used as preservative in certain foods. SO3 has a central sulfur atom and three surrounding oxygens with a total of 24 valence electrons.
Up to 24 cash back SO3 electron geometry. Moreover through the valence shell electron pair repulsion VSEPR theory the structure of sulfur trioxide SO3 is found to be bent shaped or trigonal pyramidal or trigonal planar where the bond angle is 120. What is the electron domain geometry of a molecule with 3 electron groups.
S O 3 2 Total valence electrons 6 e 3 6 e 2 e 2 6 e Formal charge on central atom 6 2 2 1 8 0. Want to see this answer and more. However the molecular geometry of SO3 looks trigonal planar shaped and zero lone pair of electron on the sulfur of the SO3 geometry.
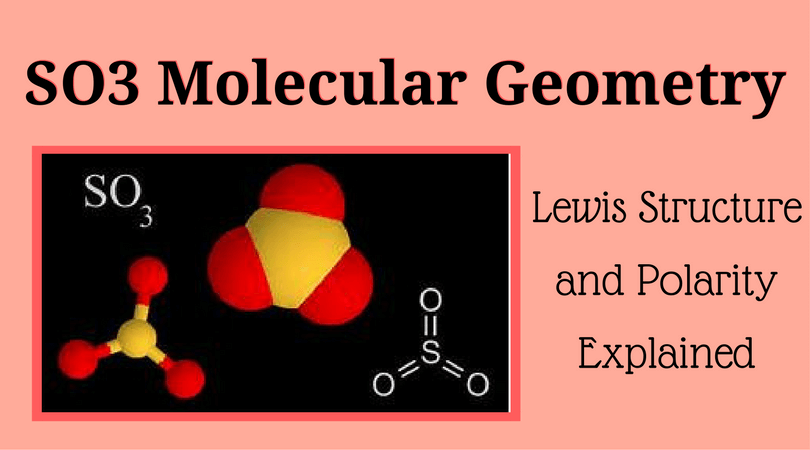
So3 Molecular Geometry Lewis Structure And Polarity Explained

So3 Molecular Geometry Shape And Bond Angles Sulfur Trioxide Youtube
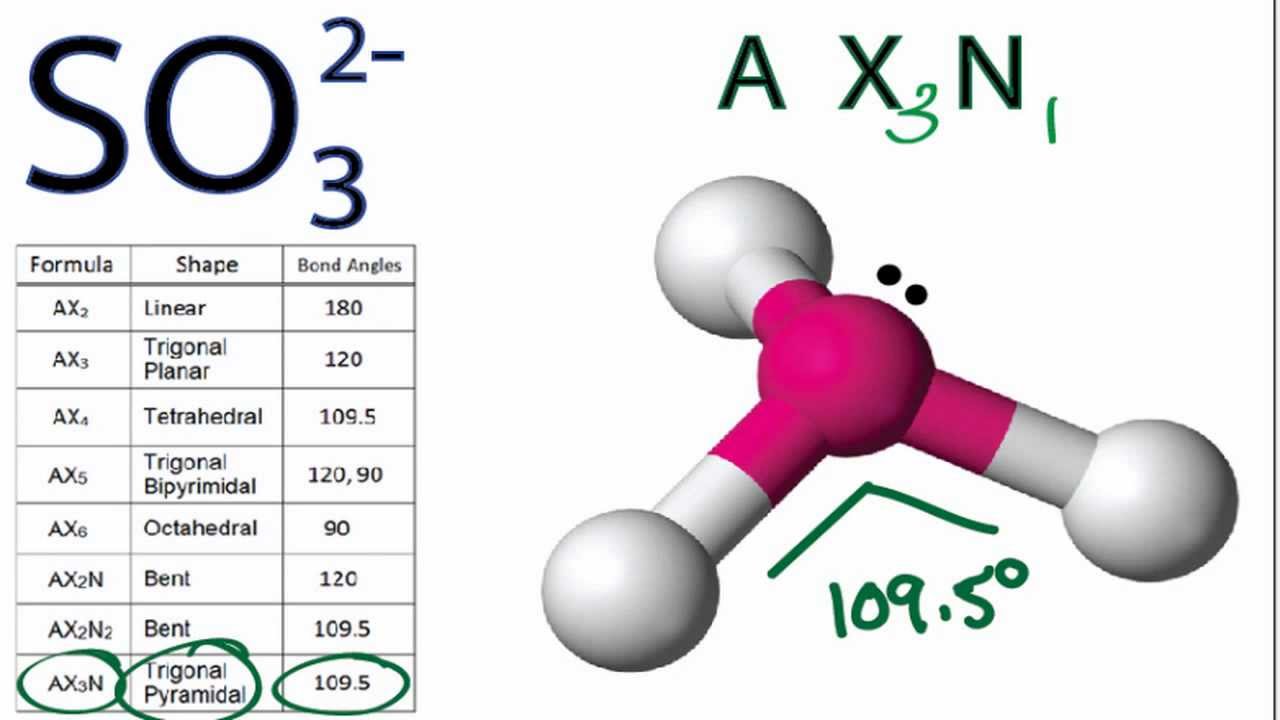
So32 Molecular Geometry Shape And Bond Angles Note Precise Angle Is 106 Degrees Youtube
No comments for "What Is the Electron Geometry of So3"
Post a Comment